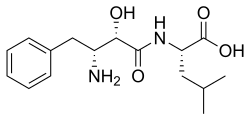
烏苯美司
外觀
 | |
 | |
| 臨床資料 | |
|---|---|
| 商品名 | 百士欣等 |
| 其他名稱 | Bestatin; N-[(2S,3R)-3-Amino-2-hydroxy-4-phenylbutyryl]-L-leucine |
| AHFS/Drugs.com | 國際藥品名稱 |
| 法律規範狀態 | |
| 法律規範 | |
| 藥物動力學數據 | |
| 藥物代謝 | 肝臟[2] |
| 生物半衰期 | 2.1±0.7小時[3] |
| 排泄途徑 | 尿[2] |
| 識別資訊 | |
| |
| CAS號 | 65391-42-6(鹽酸鹽) 58970-76-6 |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.055.917 |
| 化學資訊 | |
| 化學式 | C16H24N2O4 |
| 摩爾質量 | 308.38 g·mol−1 |
| 3D模型(JSmol) | |
| 熔點 | 245 °C(473 °F) |
| 水溶性 | 1.29(預測)[4] mg/mL (20 °C) |
| |
| |
烏苯美司(INN:Ubenimex)[5],又名貝他定(英語:Bestatin),是一種競爭性、可逆性的蛋白酶抑制劑,最初由橄欖網狀鏈黴菌(學名:Streptomyces olivoreticuli)培養濾液中分離得到[2][6][7]。
烏苯美司是以下酶的抑制劑:
- 氨基肽酶B[8]
- 白三烯A4水解酶(一種具有環氧化物水解酶和氨基肽酶活性的雙功能鋅酶)[9]
- 丙氨酸氨肽酶(氨基肽酶M/N)[10]
- 亮氨酰/半胱氨酰氨肽酶(催產素酶/抗利尿激素酶)[11][12]
- 膜二肽酶(白三烯D4水解酶)
用途
[編輯]烏苯美司正在被研究用於治療急性骨髓性白血病[13]和淋巴水腫[14]。由於烏苯美司是免疫調節劑,可增強T細胞功能[2],它也被研究作為HIV-1 DNA疫苗接種的免疫佐劑[15]。

參見
[編輯]參考文獻
[編輯]- ^ STANDARD FOR THE UNIFORM SCHEDULING OF MEDICINES AND POISONS. Federal Register of Legislation. 2020-10 [2021-08-15]. (原始內容存檔於2021-01-25).
- ^ 2.0 2.1 2.2 2.3 2.4 乌苯美司片(西安万隆). 丁香園·用藥助手. [2025-03-16].
- ^ Ueda T, Tohyama K, Wano Y, Tsutani H, Fukushima T, Iwasaki H, Urasaki Y, Gotoh N, Kimura S, Okumura E; et al. Pharmacokinetic and clinical pilot study of high-dose intermittent ubenimex treatment in patients with myelodysplastic syndrome. Anticancer Res. 1994 Sep-Oct, 14 (5B): 2093–7. PMID 7840505.
- ^ Ubenimex. [2025-03-19].
- ^ N-((2S,3R)-3-Amino-2-hydroxy-4-phenylbutyryl)-L-leucine at Sigma-Aldrich
- ^ Abe F, Alvord G, Koyama M, Matsuda A, Talmadge JE. Pharmacokinetics of bestatin and oral activity for treatment of experimental metastases 28 (1): 29–33. 1989-01 [2025-03-16]. PMC 11038206
 . PMID 2909281. doi:10.1007/BF00205797.
. PMID 2909281. doi:10.1007/BF00205797.
- ^ Bauvois, B; Dauzonne, D. Aminopeptidase-N/CD13 (EC 3.4.11.2) inhibitors: Chemistry, biological evaluations, and therapeutic prospects. Medicinal Research Reviews. January 2006, 26 (1): 88–130. PMC 7168514
 . PMID 16216010. doi:10.1002/med.20044.
. PMID 16216010. doi:10.1002/med.20044.
- ^ Umezawa, H.; Aoyagi, T.; Suda, H.; Hamada, M.; Takeuchi, T. Bestatin, an inhibitor of aminopeptidase B, produced by actinomycetes.. The Journal of Antibiotics. 1976, 29 (29): 97–99. PMID 931798. doi:10.7164/antibiotics.29.97
 .
.
- ^ Muskardin, D.T.; Voelkel, N.F.; Fitzpatrick, F.A. Modulation of pulmonary leukotriene formation and perfusion pressure by Bestatin, an inhibitor of leukotriene A4 hydrolase.. Biochemical Pharmacology. 1994, 48 (48): 131–137. PMID 8043014. doi:10.1016/0006-2952(94)90232-1.
- ^ K Sekine; H Fujii; F Abe. Induction of apoptosis by Bestatin (ubenimex) in human leukemic cell lines. Leukemia. 1999, 13 (5): 729–734. PMID 10374877. doi:10.1038/sj.leu.2401388
 .
.
- ^ Nakanishi Y, Nomura S, Okada M, Ito T, Katsumata Y, Kikkawa F, Hattori A, Tsujimoto M, Mizutani S. Immunoaffinity purification and characterization of native placental leucine aminopeptidase/oxytocinase from human placenta. Placenta. 2000, 21 (7): 628–34. PMID 10985965. doi:10.1053/plac.2000.0564.
- ^ Naruki M, Mizutani S, Goto K, Tsujimoto M, Nakazato H, Itakura A, Mizuno K, Kurauchi O, Kikkawa F, Tomoda Y. Oxytocin is hydrolyzed by an enzyme in human placenta that is identical to the oxytocinase of pregnancy serum. Peptides. 1996, 17 (2): 257–61. PMID 8801531. S2CID 28486489. doi:10.1016/0196-9781(95)02124-8.
- ^ Hirayama, Y; Sakamaki, S; Takayanagi, N; Tsuji, Y; Sagawa, T; Chiba, H; Matsunaga, T; Niitsu, Y. Chemotherapy with ubenimex corresponding to patient age and organ disorder for 18 cases of acute myelogeneous leukemia in elderly patients--effects, complications and long-term survival. Gan to Kagaku Ryoho. Cancer & Chemotherapy. 2003, 30 (8): 1113–8. PMID 12938265.
- ^ Tian, W; Rockson, S; Jiang, X; Kim, J; Begaye, A; Shuffle, EM; Tu, AB; Cribb, M; Nepiyushchikh, Z; Feroze, AH; Zamanian, RT; Dhillon, RT; Voelkel, NF; Peters-Golden, M; Kitajewski, J; Dixon, JB; Nicolls, MR. Leukotriene B4 antagonism ameliorates experimental lymphedema. Science Translational Medicine. 2017, 9 (389): eaal3920. PMID 28490670. doi:10.1126/scitranslmed.aal3920
 .
.
- ^ Sasaki S, Fukushima J, Hamajima K, Ishii N, Tsuji T, Xin KQ, Mohri H, Okuda K. Adjuvant effect of Ubenimex on a DNA vaccine for HIV-1 111 (1): 30–35. 1998-01 [2025-03-16]. PMC 1904860
 . PMID 9472658. doi:10.1046/j.1365-2249.1998.00466.x.
. PMID 9472658. doi:10.1046/j.1365-2249.1998.00466.x.
外部連結
[編輯]| 這是一篇與藥學相關的小作品。您可以透過編輯或修訂擴充其內容。 |
